![The two complexes of nickel, [Ni (CN) 4 ] 2- and [Ni (CO) 4 ], have different structures but possess same magnetic behavior. Explain. The two complexes of nickel, [Ni (CN) 4 ] 2- and [Ni (CO) 4 ], have different structures but possess same magnetic behavior. Explain.](https://static.insightsonindia.in/ncertusercontent/solutions/?domain=gF&l=PROJ13574/1520335895568280.png)
The two complexes of nickel, [Ni (CN) 4 ] 2- and [Ni (CO) 4 ], have different structures but possess same magnetic behavior. Explain.
![i) Draw the geometrical isomer of complex [Pt(NH3)2Cl2] .(ii) On the basis of crystal field theory, write the electronic configuration for d^4 ion if Δ 0<P .(iii) Write the hybridization and magnetic i) Draw the geometrical isomer of complex [Pt(NH3)2Cl2] .(ii) On the basis of crystal field theory, write the electronic configuration for d^4 ion if Δ 0<P .(iii) Write the hybridization and magnetic](https://d1hj4to4g9ba46.cloudfront.net/questions/553054_507103_ans.png)
i) Draw the geometrical isomer of complex [Pt(NH3)2Cl2] .(ii) On the basis of crystal field theory, write the electronic configuration for d^4 ion if Δ 0<P .(iii) Write the hybridization and magnetic

Ni(CO)4 Hybridisation , Geometry and Magnetic nature of Tetracarbonylnickel -coordination compounds - YouTube
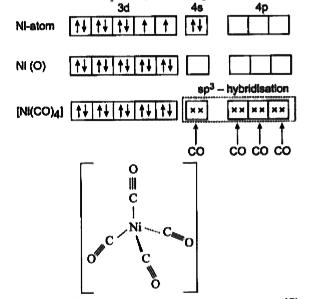
the Ni(CO)_4_ complex is diamagnetic in nature. the hybrid state of Ni is 1)sp^3 2)sp^2 d 3)sp^3d 4)spd^3
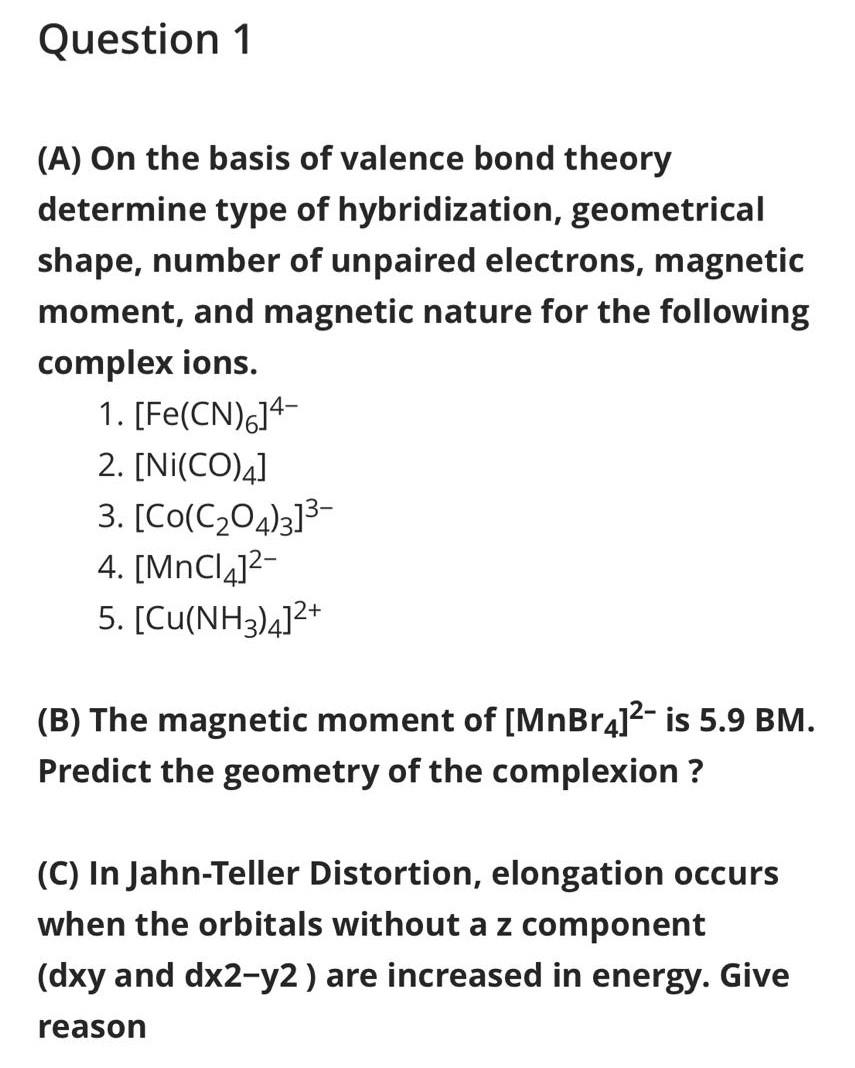
Why is Ni (CO) 4 tetrahedral and diamagnetic? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium
The geometry and magnetic behaviour of the complex [Ni(CO)4] are - Sarthaks eConnect | Largest Online Education Community

trick to identify nature of diamagnetic and paramagnetic in tetrahedral and square planner compound - YouTube
![SOLVED: "Which one of the following is paramagnetic in nature?(1) Ni(CO).(2) [Ni(CN)12-(3) K [Fe(CN) (4) [FeF]4 0da 4/9 'JaJ] (4) (2) [Ni(CN)AR?- (3) K4[Fe(CN)sl (1) Ni(CO) Which one of the following is SOLVED: "Which one of the following is paramagnetic in nature?(1) Ni(CO).(2) [Ni(CN)12-(3) K [Fe(CN) (4) [FeF]4 0da 4/9 'JaJ] (4) (2) [Ni(CN)AR?- (3) K4[Fe(CN)sl (1) Ni(CO) Which one of the following is](https://cdn.numerade.com/ask_images/c341b6dc2dd149d4af1982b2584c2e40.jpg)
SOLVED: "Which one of the following is paramagnetic in nature?(1) Ni(CO).(2) [Ni(CN)12-(3) K [Fe(CN) (4) [FeF]4 0da 4/9 'JaJ] (4) (2) [Ni(CN)AR?- (3) K4[Fe(CN)sl (1) Ni(CO) Which one of the following is
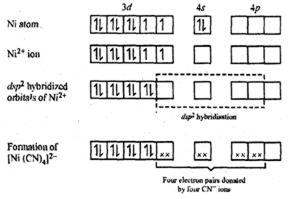
Explain the geometry of Ni(CO)4 by valence bond theory. Why is this molecule diamagnetic? (Atomic number of Ni = 28). from Chemistry Coordination Compounds Class 12 Haryana Board - English Medium
![Which one of the following is paramagnetic in nature?(1) Ni(CO).(2) [Ni(CN)12-(3) K [Fe(CN) (4) [FeF]4 - Brainly.in Which one of the following is paramagnetic in nature?(1) Ni(CO).(2) [Ni(CN)12-(3) K [Fe(CN) (4) [FeF]4 - Brainly.in](https://hi-static.z-dn.net/files/d92/6529d134f33502baa60cb05a087be920.jpg)
![NiCl4]2− is paramagnetic while [NiCO4] is diamagnetic though both are tetrahedral. Why? NiCl4]2− is paramagnetic while [NiCO4] is diamagnetic though both are tetrahedral. Why?](https://df0b18phdhzpx.cloudfront.net/ckeditor_assets/pictures/1577567/original_Coordination_compound_12_6.png)
![How many geometrical isomers are possible for the complex [Ni(CO)(4)] How many geometrical isomers are possible for the complex [Ni(CO)(4)]](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/30713892_web.png)
![How many of the following are paramagnetic in nature? [Fe(CN)6]4−,[Fe(CN.. How many of the following are paramagnetic in nature? [Fe(CN)6]4−,[Fe(CN..](https://static-images.findfilo.com/classroom/1663205489188_pgghdnif_2437271.jpg)


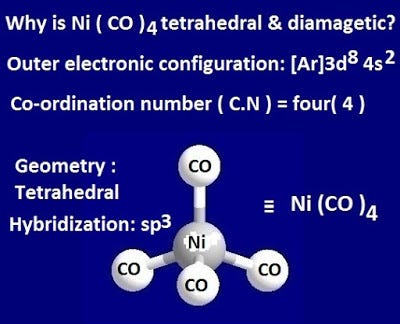
![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-3.png)

![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-1.png)



![what is the hybridization and magnetic behaviour of [Ni(CO)4] - Brainly.in what is the hybridization and magnetic behaviour of [Ni(CO)4] - Brainly.in](https://hi-static.z-dn.net/files/d20/ed425ebf73324024875c59a95f40f06c.jpg)
![NiCl(4)]^(2-) is paramagnetic while [Ni(CO)(4)] is diamgnetic though both are tetrahedral. Why ? NiCl(4)]^(2-) is paramagnetic while [Ni(CO)(4)] is diamgnetic though both are tetrahedral. Why ?](https://d10lpgp6xz60nq.cloudfront.net/physics_images/VIK_CHE_QB_C07_E04_028_S01.png)