
SOLVED: 1. Electrolysis of the NiSO4 solution for 45 minutes resulted in 9.75 g of Ni precipitate. How many grams of Ag is produced if the current is flowed at the same
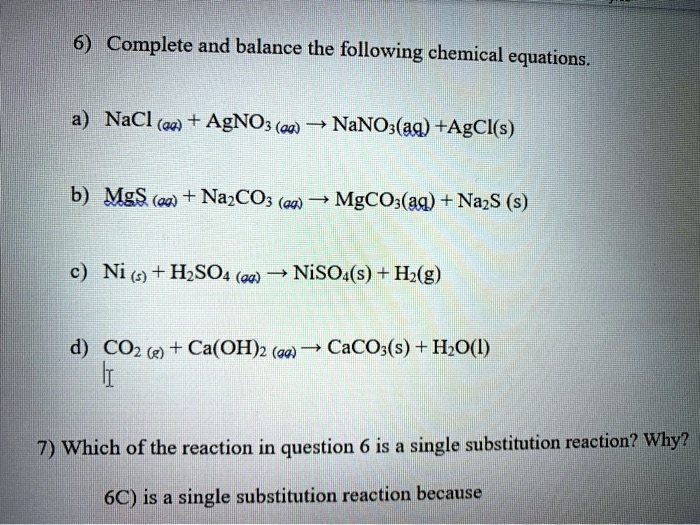
SOLVED: Complete and balance the following chemical equations. NaCl (a4) + AgNO: (aq) NaNOs(aq) +AgCl(s) b) MgS (aa) + NazCO; (a4) MgCO3(aq) + NazS (s) c) Ni () + HzSO4 (a4) NiSOa(s) +

Four Faraday of electricity were passed through aqueous solutions of `AgNO_(3), NiSO_(4), FeCl_(3)` - YouTube
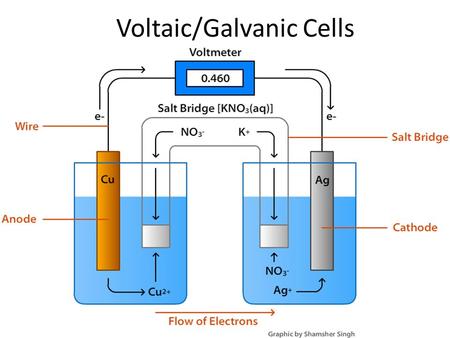
Chapter 20: Electrochemistry. © 2009, Prentice-Hall, Inc. Electrochemical Reactions In electrochemical reactions, electrons are transferred from one species. - ppt download

Гальванический элемент составлен по схеме: Ni | NiSO4 (0,1 M) || AgNO3 (0,1 M) | Ag. Напишите - Школьные Знания.com

Electroplated Silver–Nickel Core–Shell Nanowire Network Electrodes for Highly Efficient Perovskite Nanoparticle Light-Emitting Diodes | ACS Applied Materials & Interfaces
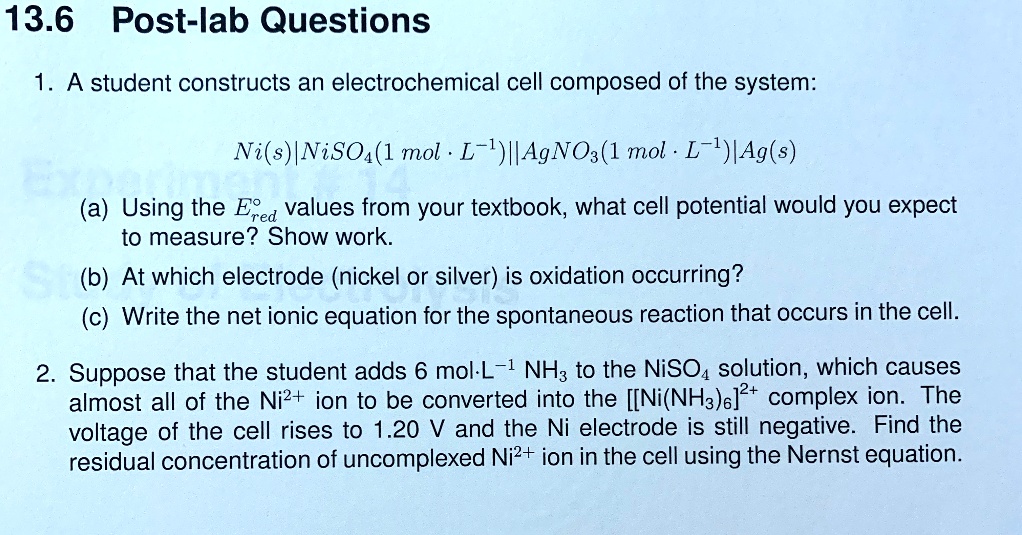
SOLVED: 13.6 Post-lab Questions student constructs an electrochemical cell composed of the system: Ni(s) NiSO4(1 mol L-')IlAgNO:(1 mol . L-1)Ag(s) (a) Using the Ered values from your textbook; what cell potential would
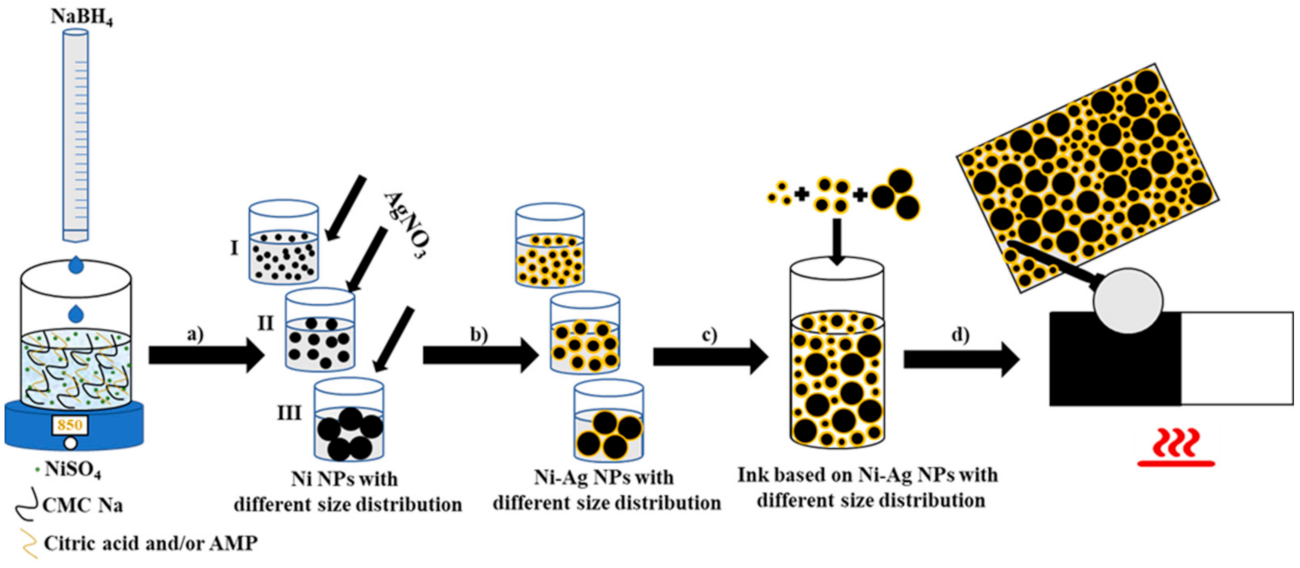
Materials | Free Full-Text | Polydispersity vs. Monodispersity. How the Properties of Ni-Ag Core-Shell Nanoparticles Affect the Conductivity of Ink Coatings
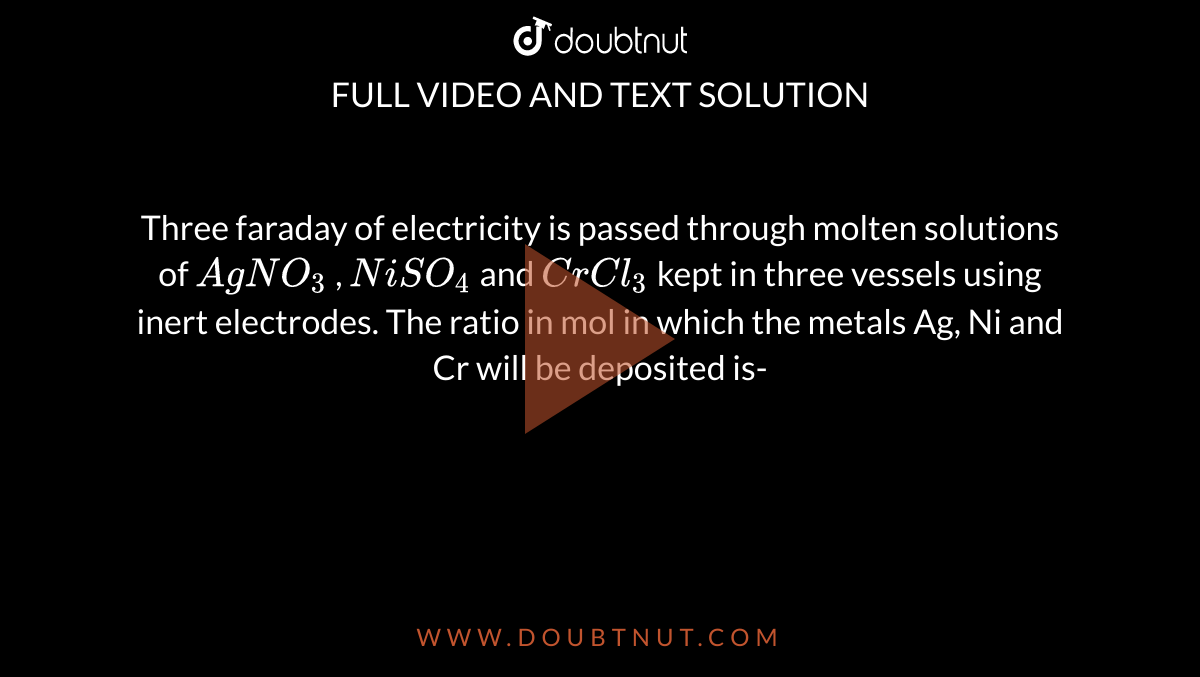
Three faraday of electricity is passed through molten solutions of AgNO3 , NiSO4 and CrCl3 kept in three vessels using inert electrodes. The ratio in mol in which the metals Ag, Ni

The composition of electrodeposited NiAg thin film with different pH... | Download Scientific Diagram

Ni-Rich LiNi0.8Co0.1Mn0.1O2 Oxide Coated by Dual-Conductive Layers as High Performance Cathode Material for Lithium-Ion Batteries | ACS Applied Materials & Interfaces
![PDF] Rapid synthesis of Ag@Ni core-shell nanoparticles using a microwave-polyol method | Semantic Scholar PDF] Rapid synthesis of Ag@Ni core-shell nanoparticles using a microwave-polyol method | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/2e161cc72b4229f5172321f287dcb084cfd990fc/9-Figure5-1.png)





![PDF] Electrodeposition of Silver-Nickel Thin Films with a Galvanostatic Method | Semantic Scholar PDF] Electrodeposition of Silver-Nickel Thin Films with a Galvanostatic Method | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/8b33b24be429a52c3e54f8d5713d82bd7bcac984/1-Figure1-1.png)





![PDF] Electrodeposition of Silver-Nickel Thin Films with a Galvanostatic Method | Semantic Scholar PDF] Electrodeposition of Silver-Nickel Thin Films with a Galvanostatic Method | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/8b33b24be429a52c3e54f8d5713d82bd7bcac984/2-Figure3-1.png)
